Discovered almost 200 years ago, the phosphomolybdenum blue (PMoB) method has undergone countless optimizations and modifications, reported in over 2500 publications. Yet findings of these works are incomplete and sometimes contradictory. The recently published, outstanding comprehensive review of PMoB method and its underlying chemistry, entitled "The molybdenum blue reaction for the determination of orthophosphate revisited: Opening the black box" (Nagul 2015), summarizes all what we know about this method. This review also inspires further questions, such as:
1) How efficient has been the optimization of PMoB method, in absence of data on initial reaction rates, and without spectra of reaction products in presence and also absence of phosphate?
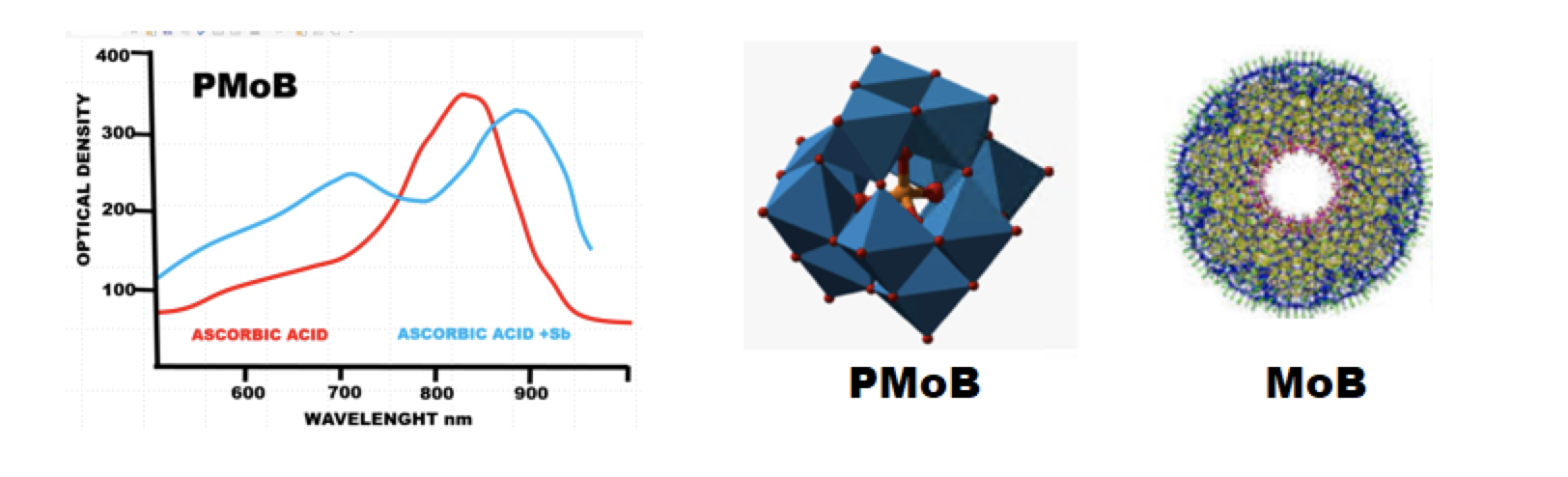
2) Why reported molar absorptivity (e) obtained with the same procedure (Murphy-Riley) varies from 21.600 AU to 25.670 AU while the highest reported (e) value at nearby wavelength is 32.000 AU (Nagul 2015 Table 3)
3) While 880nm is most frequently used wavelength, why almost any wavelength within 500nm to 900nm range was used as well?
4) While it is generally accepted the PMoB assay is best performed in acid solutions pH 0 to 1, what are the reasons for this method to fail outside this range?
5) While it is known that suitable concentration of molybdate and acidity of solution are mutually dependent, is it their ratio suitable as a guide for optimization?
6) While sulfuric acid has been most frequently used , it is not clear why other strong acids were only seldom used, although use of nitric acid was reported by Linarez (1986) and use of hydrochloric acid would be advantageous, for determination of traces of phosphate, because hydrochloric acid is available in high purity .
It is indeed justified to depict PMoB and MoB chemistry as a black box, the contents of which needs further investigation. In the following sections, pFI programmed to function in a batch mode, will be used to answer the above questions, and to optimize conditions for trace analysis of phosphate, by identifying conditions at which the slope of calibration line is maximized.